A spectrophotometer consists of two instruments, namely a spectrometer for producing light of any selected color (wavelength), and a photometer for measuring the intensity of light. The instruments are arranged so that liquid in a cuvette can be placed between the spectrometer beam and the photometer. The amount of light passing through the tube is measured by the photometer. The photometer delivers a voltage signal to a display device, normally a galvanometer. The signal changes as the amount of light absorbed by the liquid changes.
If development of color is linked to the concentration of a substance in solution then that concentration can be measured by determining the extent of absorption of light at the appropriate wavelength. For example hemoglobin appears red because the hemoglobin absorbs blue and green light rays much more effectively than red. The degree of absorbance of blue or green light is proportional to the concentration of hemoglobin.
When monochromatic light (light of a specific wavelength) passes through a solution there is usually a quantitative relationship (Beer's law) between the solute concentration and the intensity of the transmitted light, that is,
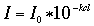
where I sub 0 is the intensity of transmitted light using the pure solvent, I is the intensity of the transmitted light when the colored compound is added, c is concentration of the colored compound, l is the distance the light passes through the solution, and k is a constant. If the light path l is a constant, as is the case with a spectrophotometer, Beer's law may be written,
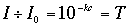 where k is a new constant and T is the transmittance of the solution. There is a logarithmic relationship between transmittance and the concentration of the colored compound. Thus,
where k is a new constant and T is the transmittance of the solution. There is a logarithmic relationship between transmittance and the concentration of the colored compound. Thus,
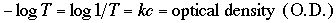 The O.D. is directly proportional to the concentration of the colored compound. Most spectrophotometers have a scale that reads both in O.D. (absorbance) units, which is a logarithmic scale, and in % transmittance, which is an arithmetic scale. As suggested by the above relationships, the absorbance scale is the most useful for colorimetric assays.
The O.D. is directly proportional to the concentration of the colored compound. Most spectrophotometers have a scale that reads both in O.D. (absorbance) units, which is a logarithmic scale, and in % transmittance, which is an arithmetic scale. As suggested by the above relationships, the absorbance scale is the most useful for colorimetric assays.
![]()
No comments:
Post a Comment